Difference between revisions of "Promoter Analysis"
(→JASPAR CORE database) |
m (→TF Mapping) |
||
| Line 24: | Line 24: | ||
[[Image:T_Promoter_Logo_TBP.png]] | [[Image:T_Promoter_Logo_TBP.png]] | ||
| − | ==TF Mapping== | + | ==TF Mapping tab== |
===List of available transcription factors=== | ===List of available transcription factors=== | ||
Revision as of 12:30, 14 October 2009
|
Home | Quick Start | Basics | Menu Bar | Preferences | Component Configuration Manager | Workspace | Information Panel | Local Data Files | File Formats | caArray | Array Sets | Marker Sets | Microarray Dataset Viewers | Filtering | Normalization | Tutorial Data | geWorkbench-web Tutorials |
Analysis Framework | ANOVA | ARACNe | BLAST | Cellular Networks KnowledgeBase | CeRNA/Hermes Query | Classification (KNN, WV) | Color Mosaic | Consensus Clustering | Cytoscape | Cupid | DeMAND | Expression Value Distribution | Fold-Change | Gene Ontology Term Analysis | Gene Ontology Viewer | GenomeSpace | genSpace | Grid Services | GSEA | Hierarchical Clustering | IDEA | Jmol | K-Means Clustering | LINCS Query | Marker Annotations | MarkUs | Master Regulator Analysis | (MRA-FET Method) | (MRA-MARINa Method) | MatrixREDUCE | MINDy | Pattern Discovery | PCA | Promoter Analysis | Pudge | SAM | Sequence Retriever | SkyBase | SkyLine | SOM | SVM | T-Test | Viper Analysis | Volcano Plot |
Contents
Overview
The Promoter component scans one or more sequence profiles against nucleotide sequences that the user has loaded into geWorbench. Motifs from the JASPAR database of transcription factor binding sites are included with the component. Additional motifs can be added by the user.
The Promoter component will also display the results of hits found in the Pattern Discovery component.
JASPAR CORE database
The promoter component of geWorkbench includes version 3.0 of the JASPAR CORE database (http://jaspar.genereg.net/). It contains 138 curated, non-redundant profiles. These "profiles are derived from published collections of experimentally defined transcription factor binding sites for multi-cellular eukaryotes. The database represents a curated collection of target sequences" (JASPAR Documentation).
The datafile used "MATRIX_DATA.txt" can be found at http://jaspar.genereg.net/html/DOWNLOAD/mySQL/JASPAR_CORE_2008/.
The profiles represent counts of how many times each of the four nucleotide bases occurs at a particular position in the aligned promoter sequences.
Working with the Promoter graphical interface
Layout
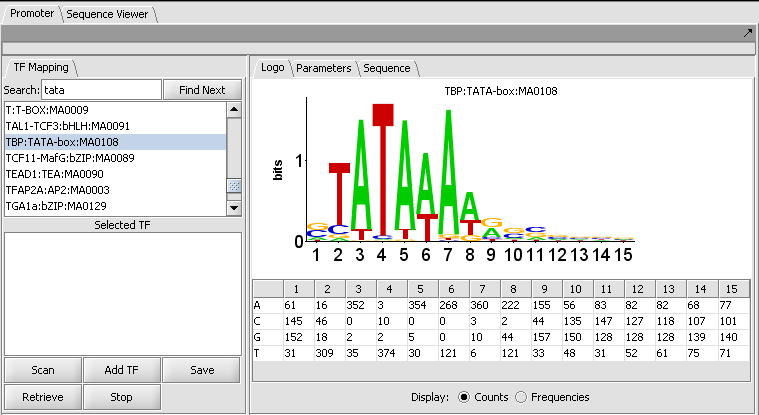
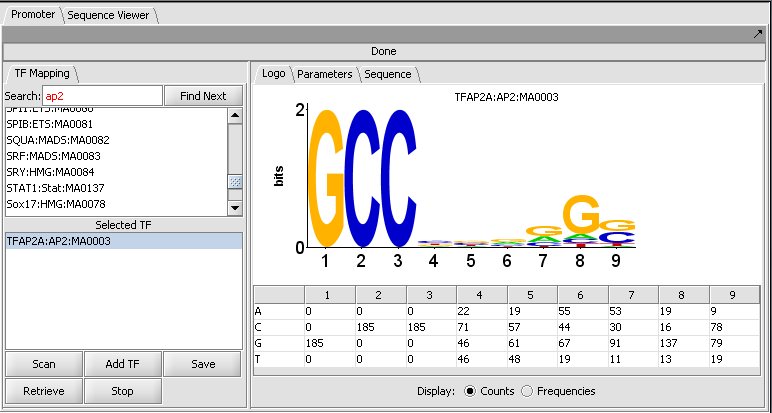
The figure shows the display of profile "TBP:TATA-box:MA0108" from the list of those included in JASPAR.
TF Mapping tab
List of available transcription factors
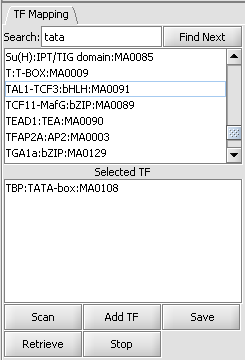
This list contains all loaded transcription factor profiles, including those from JASPAR and any loaded by the user. A profile can be transferred to the "Selected TF" list just below by double-clicking on its entry. Multiple profiles can be moved to the selected list.
- Search - search the list items for the input characters strings.
- Find Next - continue scanning the list for the next occurrence of the search string.
Selected TF
This list contains the profiles that will be used in the next scan. Entries on the Selected TF list can be moved back up to the "available TFs" list by again double-clicking on their entry.
Here the TATA-box entry has been moved to the selected list.
Controls
- Scan - start the scan of the current nucleotide sequence with the selected profiles.
- Add TF - load a new profile from a file into the list of available profiles. This is not a permanent addition; it remains loaded only for the current invocation of geWorkbench. See the "Profile File Format" entry below for details.
- Save - Saves a list of hits by a profile to a nucleotide sequence, including the sequence identifier and the start and stop points of the match along the sequence, as shown here:
gi|65508003
ATHB5:HOMEO-ZIP:MA0110 2104 2113
ATHB5:HOMEO-ZIP:MA0110 2115 2106
ATHB5:HOMEO-ZIP:MA0110 2882 2891
ATHB5:HOMEO-ZIP:MA0110 2893 2884
- Retrieve - Not implemented.
- Stop - Stop the current scan.
Profile file format
A profile in the form of a count matrix can be loaded from an external file. The profile should consist of a tab delimited series of counts, one for each position in the profile. It should could four lines, in the order A, C, G, T. There are no header lines or row labels, just the numeric matrix. For example, here is a profile showing the first six columns:
0 12 0 0 1 0
49 0 20 23 3 45
0 37 29 2 45 4
0 0 0 25 0 0
Because the normalization step uses the count total (sequences aligned to generate the profile), loading a frequency matrix is not currently supported.
The LOGO tab
LOGO display
The LOGO display implements the method of Schneider and Stephens (1990) to display the information at each position in a motif. Briefly, the total height of the column of letters at a position shows the information available, on a scale of 0 to 2 bits (the information needed to represent the 4 possible nucleotide bases at each position). The relative heights of each letter in a column show their individual contribution to the information at that position.
The LOGO display in geWorkbench implements the "small sample correction" described by Schneider, the magnitude of which depends on the number of sequences aligned to generate the profile. The correction is subtracted from the calculated information content at each position, with a minimum value (floor) of zero being displayed.
Table display
A table is used to show the numeric data from which the LOGO diagram is generated. The table depicts each position in the profile as a column, and has a row for each of the four nucleotide bases A, C, G and T. The user can choose to display the data either as the original counts or as frequencies.
- Display: Counts or Frequencies
The Parameters tab
Background sequence and scoring threshold determination
A background sequence is used to estimate an appropriate scoring threshold. This background can be generated in two ways.
- determine base composition of input sequence and from this generate random sequence.
- (13K) - use a set of 13,000 promoter sequences as background.
- The length of background sequence scanned is given by 1000*Iterations/PValue.
- The threshold value is calculated by scanning the background sequence with the profile and finding the top 100 scores. The 100th score is used as the threshold.
- Calculated p-values are Bonferroni corrected and also corrected for duplicates in the list of 100 top scores.
- Positive and negative strands are scanned and values above threshold are reported.
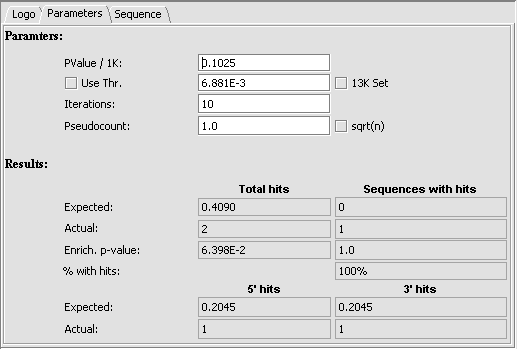
Parameters
- PValue / 1K -
- Use Thr. - Use threshold - if checked, use a user-input threshold rather than a calculated threshold for scoring a match.
- 13K Set - If not checked (default), use the random background sequence described above. If checked, use the 13K sequences as background.
- Iterations -
- Pseudocount - a small-sample correction factor (default 1.0). See above description.
Results
Total hits and Sequences with hits
Total hits counts all hits regardless of how many times one sequence is hit.
- Expected - number of hits expected by chance.
- Actual - observed number of hits.
- Enrich. p-value - p-value for chance of getting this outcome by chance.
- % with hits
5' hits and 3'hits
- Expected - Expected number of hits
- Actual - Actual number of hits.
The Sequence tab
Scan Implementation
Normalization and the Pseudocount
The count matrices are normalized to frequencies using an algorithm which includes a "pseudocount" (see Nishida 2009). The pseudocount is a way to compensate for the effects of small sample sizes in the original observations used to generate the profiles. Nishida et al. studied how to determine an appropriate value for the pseudocount. They found that the optimal values were independent of the sample size and were correlated with the entropy of the original matrices. They say that this implies that the less-conserved the binding site, the larger a value should be used for the pseudocount. They find that 0.8 is a good value "for practical uses". They do not recommend use of the square root of the total count.
geWorkbench allows a pseudocount factor to be directly entered, or it can be selected to be the square root of the total count of sequences used to generate the profile. Prior to geWorkbench 1.8.0, setting the pseudocount to the square root of the total counts was directly coded and not changeable. The current default is to set the pseudocount equal to 1.0.
The normalization forumula used in calculating frequencies is then, where b is the pseudocount, and counts(i, j) is the observed count in a particular entry in the matrix,
freq(i, j) = (counts(i, j) + b/4) / (totalCounts + b).
The resulting frequency matrix is used in the subsequent scan.
Because the pseudocount is a settable parameter, the frequency matrix is recalculated for each scan from the original counts.
Scoring
- Calculated p-values are Bonferroni corrected and also corrected for duplicates in the list of 100 top scores found during the background scan.
- Positive and negative strands are scanned and values above threshold are reported.
Running and viewing a scan
Prerequisites
The Promoter component is only available when a sequence has been loaded, either from disk or for example using the Sequence Retriever component to obtain genomic sequence.
For this example, we will obtain upstream genomic sequence for CDH2, the N-Cadherin gene. However, using the Sequence Retriever component requires that a microarray dataset and its annotations be loaded. Here, we will use the JB-ccmp_0120.txt file, which is an Affymetrix HG-U95Av2 MAS5 format text file and is part of the geWorkbench tutorial dataset.
The Affymetrix HG-U95Av2 annotation file can be obtain from the Affymetrix website. Please see instructions on the geWorkbench FAQ.
1. In the Project Folders component, load the file JB-ccmp_0120.txt as type MAS5/GCOS.
2. When prompted, associate the HG-U95Av2 annotation file.
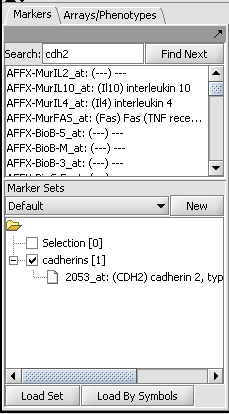
3. In the Markers component, search for the gene name "CDH2" using the Find Next button. On this chip type, marker 2053_at represents the CDH2 gene.
4. You can double-click on the marker to add it to the default "Selection" set. Or you can right-click on it and add it to a named set, such as "Cadherins". This is depicted below.
5. "Activate" (check the box next to) the set to which you added the marker.
6. Any activated markers will appear in the Sequence Retriever component, as shown below.
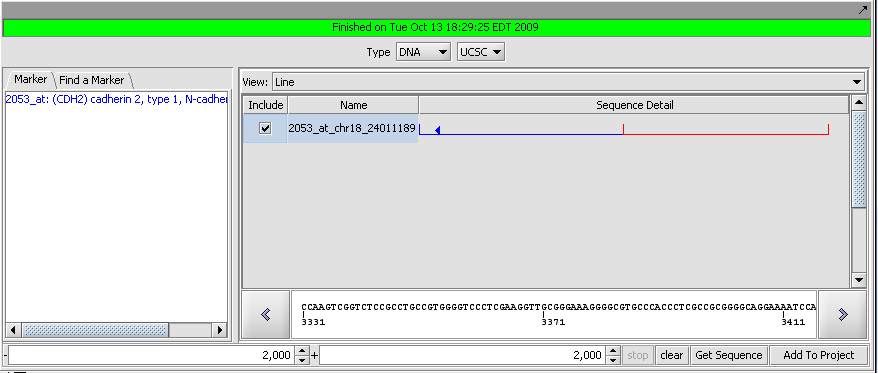
7. Set the retrieval limits to 2000 base pairs up- and downstream from the transcription start site.
8. Make sure the retrieval type is set to DNA, UCSC (the Santa Cruz genome sequence database).
9. Click "Get Sequence".
10. Once the sequence has been retrieved, check the box next to the sequence and then hit the button "Add to Project". We now have the genomic sequence available for other components to use.
Running a promoter scan
1. In the Promoter component, search for "ap2", which corresponds to transcription factor activator protein 2 alpha.
2. Double-click on the TFAP2A entry to move it down to the search list.
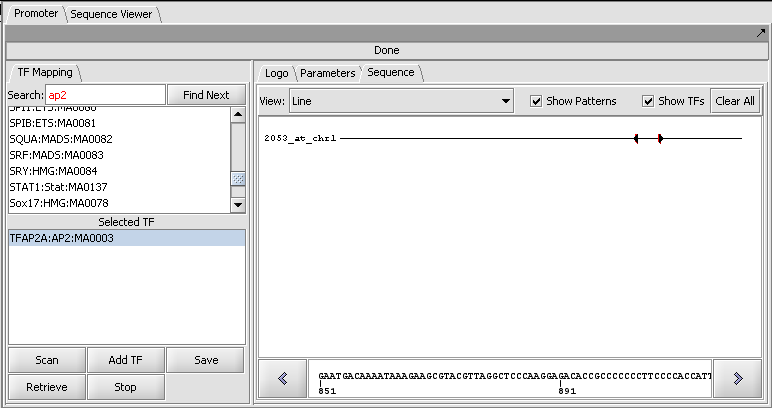
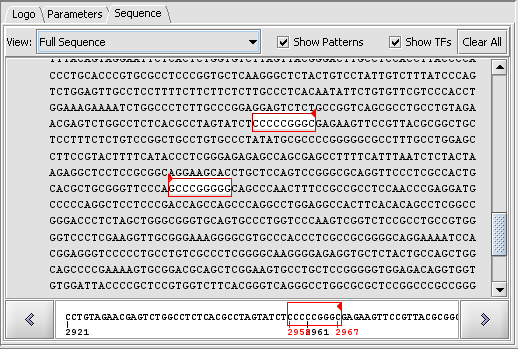
3. Hit the "Scan" button. The result is displayed in the Sequence tab of the Promoter component as shown here.
4. Setting the View to Full Sequence shows the hits in white on the sequence. Red arrows indicate whether the hit is to the forward (right arrow) or reverse (complementary) (left-arrow) strand.
5. The parameters tab displays the actual threshold values calculated during the run, and displays the enrichment results.
References
- Nishida K, Frith MC, Nakai K. (2009) Pseudocounts for transcription factor binding sites. Nucleic Acids Res. Feb;37(3):939-44. link to paper
- Sandelin A, Alkema W, Engstrom P, Wasserman WW, Lenhard B. (2004) JASPAR: an open-access database for eukaryotic transcription factor binding profiles. Nucleic Acids Res. Jan 1;32(Database issue):D91-4 (link to paper).
- Schneider TD, Stephens RM. (1990) Sequence logos: a new way to display consensus sequences. Nucleic Acids Res. Oct 25;18(20):6097-100. (link to paper)
- Vlieghe D, Sandelin A, De Bleser PJ, Vleminckx K, Wasserman WW, van Roy F, Lenhard B. (2006) A new generation of JASPAR, the open-access repository for transcription factor binding site profiles. Nucleic Acids Res. Jan 1;34(Database issue):D95-7.